Publication List
Prof. Kaoru Dokko
Prof. Ryoichi Tatara
Dr. Hisashi Kokubo
Materials Science Related to Electrochemical Devices
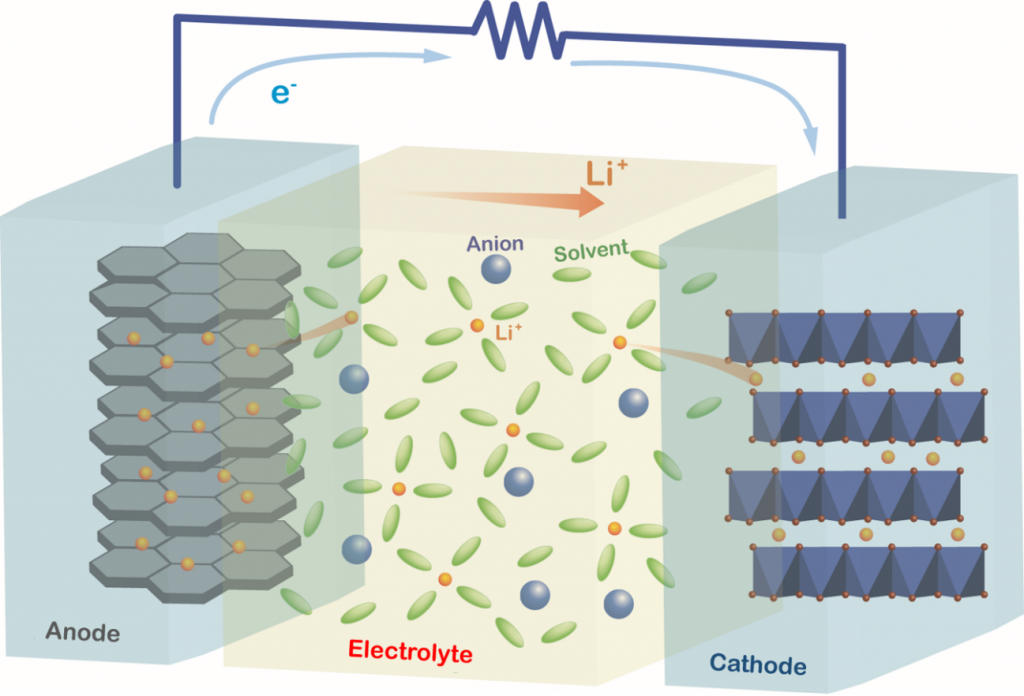
In our research group, we are investigating the structures, physicochemical properties, and electrochemical reactions of materials for electrochemical devices. We are involved in the research and development of liquid electrolytes, polymer-gel electrolytes, solid-state electrolytes, electrode materials, and other functional materials for Li-ion batteries (LIBs) as well as next-generation batteries, such as Li-S and Na-ion batteries.
Novel Electrolytes
We aim to develop novel electrolytes for next-generation batteries. For instance, we are developing ionic liquids and molten solvates as electrolytes for LIBs and Li-S batteries. Ionic liquids are non-flammable and non-volatile, and the utilization of an ionic liquid electrolyte may be beneficial in achieving high thermal stability in batteries. To understand the physicochemical properties of liquid electrolytes, we are investigating the liquid structures and interactions between cations and anions using various experimental techniques. In liquid electrolytes, cations and anions form ion-pairs and ionic aggregates, and the liquid structure significantly affects the ion conduction, thermal properties, and electrochemical properties of the electrolytes. Therefore, fundamental studies are crucial for the development of new electrolytes for next-generation batteries. Along with liquid electrolytes, other electrolytes, such as polymer gel and composite (composed of inorganic solid electrolytes and polymer electrolytes) electrolytes have also been studied.
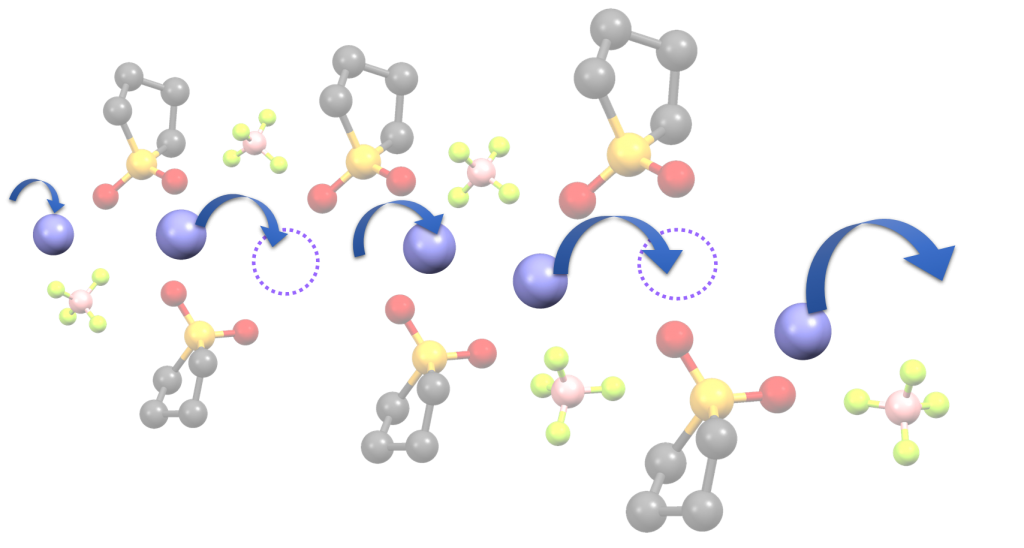
(K. Dokko et al., J. Phys. Chem. B, 2018, 122, 10736-10745)
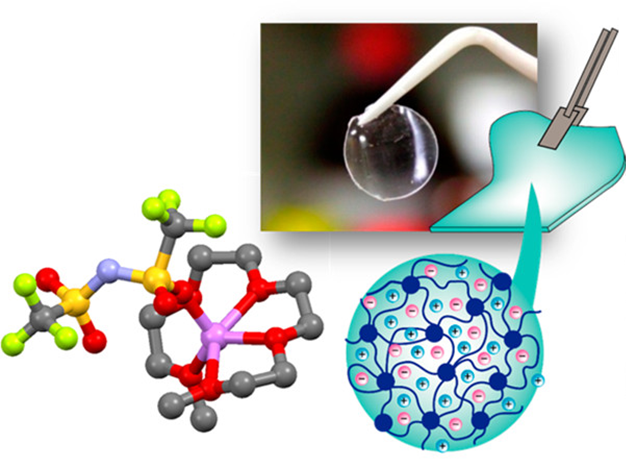
(Y. Kitazawa et al., Chem. Mater. 2018, 30, 252–261.)
Electrochemical Reaction Mechanisms in Batteries
During the charging and discharging processes of a battery, electrochemical reactions occur simultaneously with the charge transfer process at the electrode/electrolyte interface and the mass transport process in the electrolyte. For example, the Li-ion transfer process at the electrode/electrolyte interface involves the desolvation of Li ions; moreover, the interaction between the Li ion and solvent significantly affects the electrochemical reaction mechanism. In addition to the interfacial process, the mass transport process in the electrolyte also influences the electrochemical reaction rate. The Li-ion diffusion in the vicinity of the electrode and the Li-ion migration in the bulk of the electrolyte become rate-determining steps at high current densities. Understanding the thermodynamic and kinetic factors and the mass transport processes, and interpreting their effects on the electrochemical reactions is crucial for designing electrochemical devices. We aim to elucidate the mechanisms of the interfacial charge transfer reactions and ion transport in batteries using electrochemical methods.
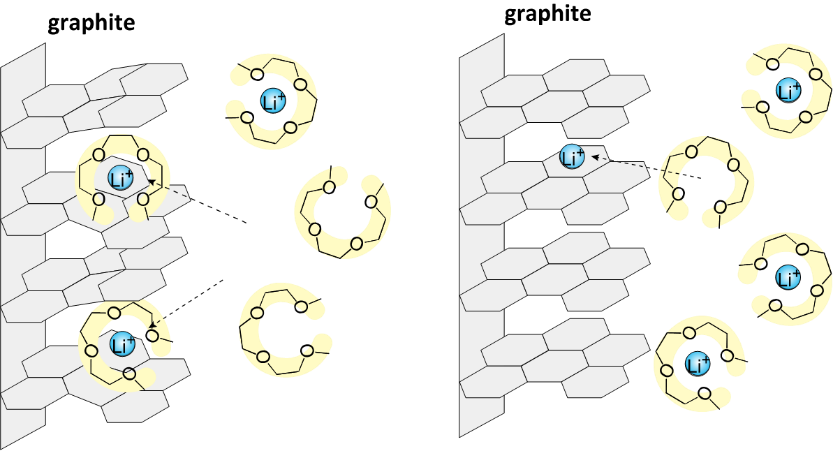
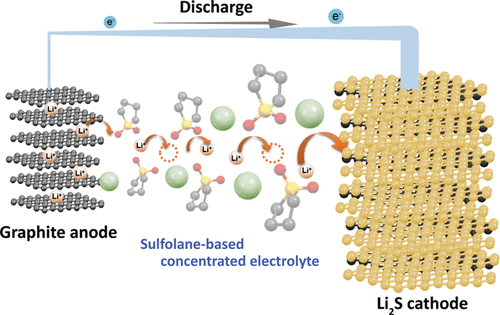
(T. Seita et al., ACS Energy Lett. 2020, 5, 1-7.)
